
Cooking 101: What happens when you put oil into a pot of water? Well, as any pasta lover should know, the oil floats to the surface creating its own layer that rests right above the still water beneath. But why is that?
The answer to this question, my friends, is density.
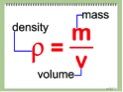 Density, in the simplest of terms, is how much of something an object has. More technically, it represents how much mass an object contains, with respect to its overall volume. Most of us are familiar with the mathematical formula for density, D=M/V, which helps to visually show us this relationship. Typically, larger, heavier objects hold more mass, where as smaller, lighter ones hold less. Comparing that to the size, or volume, of the object will determine its overall density. This will also help to judge how they may react in different mediums, such as water.
Density, in the simplest of terms, is how much of something an object has. More technically, it represents how much mass an object contains, with respect to its overall volume. Most of us are familiar with the mathematical formula for density, D=M/V, which helps to visually show us this relationship. Typically, larger, heavier objects hold more mass, where as smaller, lighter ones hold less. Comparing that to the size, or volume, of the object will determine its overall density. This will also help to judge how they may react in different mediums, such as water.
 If we cycle back to our original question as to why oil floats above water, we can see that the oil must be lighter than the water for it to rest on the surface. In fact, oil is lighter, or less dense, than our medium, which is more dense, thus explaining the phenomenon that we observe when cooking.
If we cycle back to our original question as to why oil floats above water, we can see that the oil must be lighter than the water for it to rest on the surface. In fact, oil is lighter, or less dense, than our medium, which is more dense, thus explaining the phenomenon that we observe when cooking.
Within the marine world, this concept applies to all objects that come in contact with the water. If it contains more mass than its overall volume, the object will sink and vice versa. If you drop a strawberry into the water, which actually weighs less in comparison to the amount of space it takes up for itself, it will float! As for a piece of clay, its mass is heavier, meaning that it will sink. On a more complex scale, these objects might react differently within the oceans that surround us. Because marine waters contain a higher salinity than freshwater, our strawberry and piece of clay will decrease in density, as salt water is more dense. As a result, our strawberry will continue to float to the top while our piece of clay may surface as well instead of sink.
Written by: John Cornett


